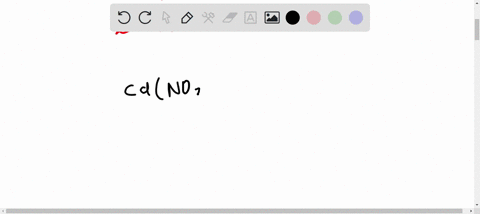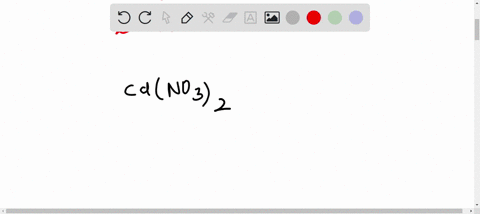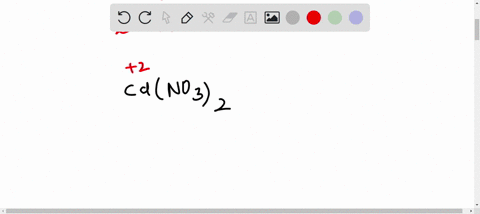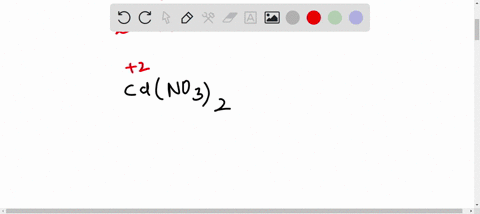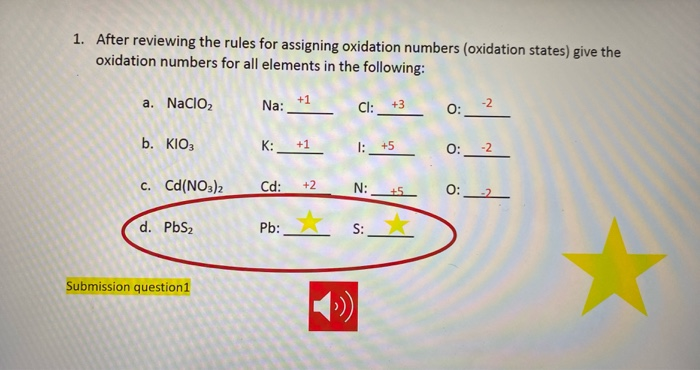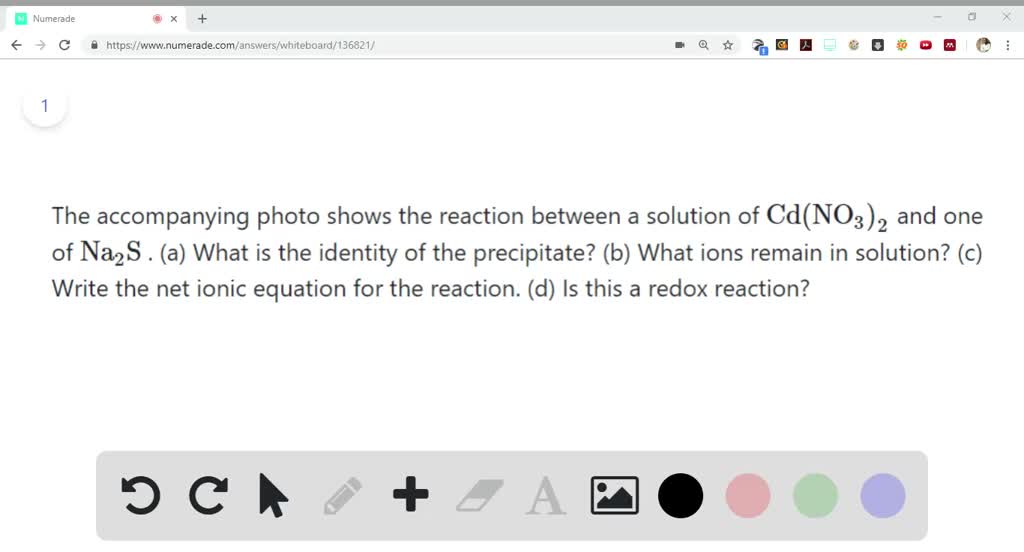
SOLVED:The accompanying photo shows the reaction between a solution of Cd( NO3)2 and one of Na2 S . (a) What is the identity of the precipitate? (b) What ions remain in solution? (c)
![Reaction of Cd(NO3)2·4H2O with 4,4'–bipyridine (bpy) in MeOH solvent: synthesis and characterization of T-shaped [Cd(bpy)1.5(NO3)2]·3H2O, square grid [Cd(bpy)2(H2O)2](NO3)2·4H2O and linear polymeric [Cd(bpy)(H2O)2(NO3)2] - ScienceDirect Reaction of Cd(NO3)2·4H2O with 4,4'–bipyridine (bpy) in MeOH solvent: synthesis and characterization of T-shaped [Cd(bpy)1.5(NO3)2]·3H2O, square grid [Cd(bpy)2(H2O)2](NO3)2·4H2O and linear polymeric [Cd(bpy)(H2O)2(NO3)2] - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1463018400000241-gr3.gif)
Reaction of Cd(NO3)2·4H2O with 4,4'–bipyridine (bpy) in MeOH solvent: synthesis and characterization of T-shaped [Cd(bpy)1.5(NO3)2]·3H2O, square grid [Cd(bpy)2(H2O)2](NO3)2·4H2O and linear polymeric [Cd(bpy)(H2O)2(NO3)2] - ScienceDirect
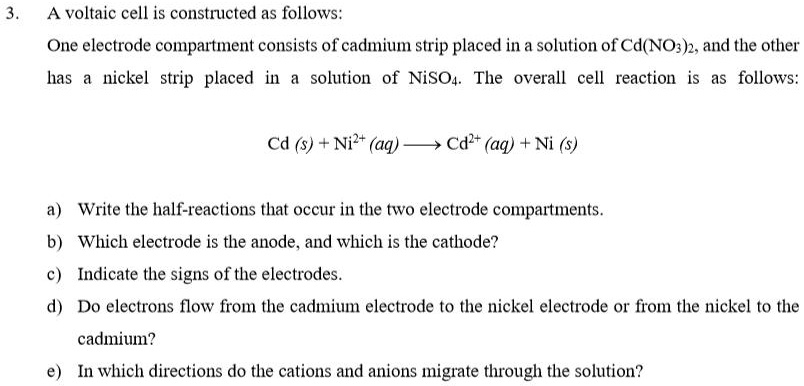
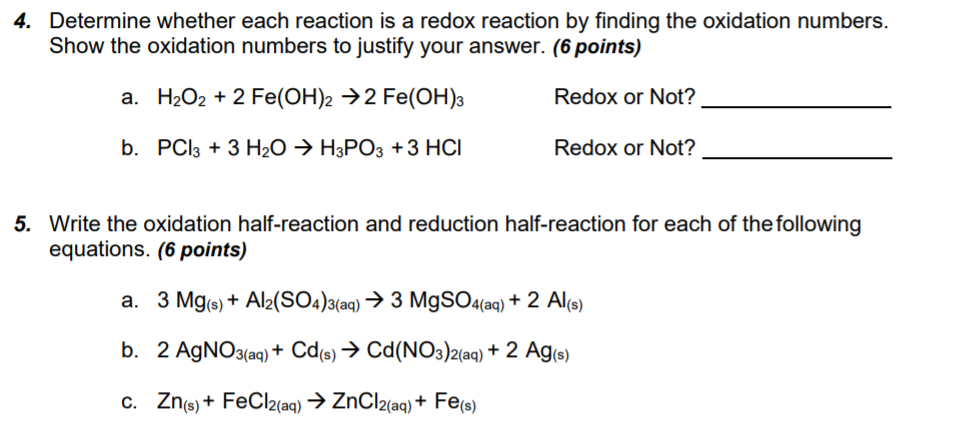
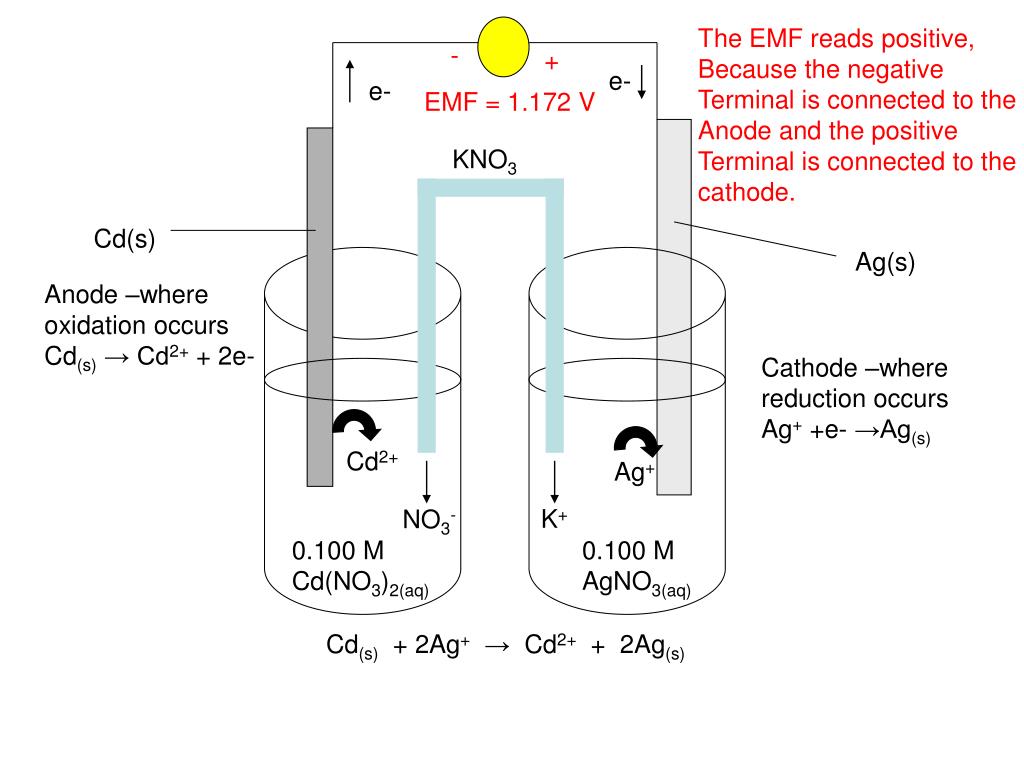
SOLVED: A voltaic cell is constructed as follows: One electrode compartment consists of a cadmium strip placed in a solution of Cd(NO3)2, and the other has a nickel strip placed in a

Which of the following is(are) incorrect statement(s)? (Assuming oxidation number of metal does not affect crystal field energy)
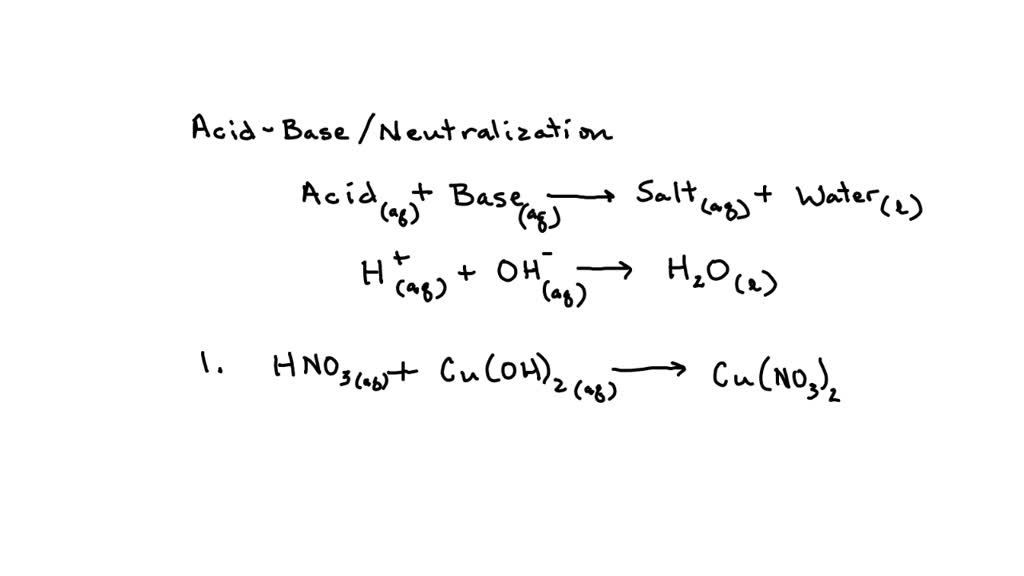
SOLVED: 1. Cadmium Nitrate + Ammonium Hydroxide Balanced equation: Cd(NO3)2 + 2NH4OH -> Cd(OH)2 + 2NH4NO3 Complete ionic equation: Cd2+(aq) + 2NO3-(aq) + 2NH4+(aq) + 2OH-(aq) -> Cd(OH)2(s) + 2NH4+(aq) + 2NO3-(aq)

Coordination of Cd(NO3)2.4H2O and Biphenyl tricarboxylic acid by using... | Download Scientific Diagram
![Reactivity of a Cadmium–Terpyridine Complex: [Cd(L1)(NO3)2(H2O)] (L1 = (4′‐(4‐bromophenyl)‐2,2′:6′,2″‐terpyridine) - Lee - 2021 - Bulletin of the Korean Chemical Society - Wiley Online Library Reactivity of a Cadmium–Terpyridine Complex: [Cd(L1)(NO3)2(H2O)] (L1 = (4′‐(4‐bromophenyl)‐2,2′:6′,2″‐terpyridine) - Lee - 2021 - Bulletin of the Korean Chemical Society - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/d3412039-c70f-4928-b764-18943f32798f/bkcs12233-fig-0012-m.jpg)
Reactivity of a Cadmium–Terpyridine Complex: [Cd(L1)(NO3)2(H2O)] (L1 = (4′‐(4‐bromophenyl)‐2,2′:6′,2″‐terpyridine) - Lee - 2021 - Bulletin of the Korean Chemical Society - Wiley Online Library
![Reaction of Cd(NO3)2·4H2O with 4,4'–bipyridine (bpy) in MeOH solvent: synthesis and characterization of T-shaped [Cd(bpy)1.5(NO3)2]·3H2O, square grid [Cd(bpy)2(H2O)2](NO3)2·4H2O and linear polymeric [Cd(bpy)(H2O)2(NO3)2] - ScienceDirect Reaction of Cd(NO3)2·4H2O with 4,4'–bipyridine (bpy) in MeOH solvent: synthesis and characterization of T-shaped [Cd(bpy)1.5(NO3)2]·3H2O, square grid [Cd(bpy)2(H2O)2](NO3)2·4H2O and linear polymeric [Cd(bpy)(H2O)2(NO3)2] - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1463018400000241-gr1.gif)